Scenario 1: For 33 years I have had nipples and never once has anything come from them and, quite frankly, they have proven to be quite useless. Then I get pregnant, notice my breast get a little larger, nipples change color and change slightly in shape. But still, they’re quite useless and nothing ever leaks from them. This crying, tiny, hungry baby comes into the world who I have to keep alive. And I’m suppose to do that with only these useless nipples that I have never once seen anything come out of. Why would I trust that they can keep this hungry baby alive?
Scenario 2: same set up: 33 years of useless nipples then I get pregnant. Then at 37 weeks pregnant, I’m in the warm shower and I try hand expression for the first time. It takes a few minutes of seeing nothing – after all, it is my first time trying and most things takes practice to figure out. Then all of the sudden: there is a drop of colostrum! I keep expressing and it keeps flowing. I DO have breastmilk in there, these nipples ARE useful. A few weeks later my hungry baby latches and not only am I confident that there is colostrum in my breasts, but I am confident and comfortable hand expressing some colostrum to give to her after she nursed, or to help her latch, or to relieve my engorgement that first week.
Scenario 1 is the sentiment that I heard many moms express those first two days in the hospital.
Scenario 2 was my personal journey. I had been in the lactation field for 5 years prior to having my first child and even I was amazed when I saw those first drops of colostrum. Imagine being a first time mom who has had no experience with breastfeeding. This is why I find antenatal (prenatal) breastmilk expression so intriguing- I think it can be so empowering and set a mom up for a confident breastfeeding journey. Now the question is: is this research based or professional opinion?
Now this article is a Scoping Review …. Which I’ve come to learn means that it can tell me all of the studies out there regarding this topic: antenatal (prenatal) breastmilk expression. But, it does not address a specific question and it does not give me one combined result. Instead, it appears to give a good overview of what research has already been done on the subject.
The authors found 20 studies to include in this scoping review, ranging from 1946-2019. After critically appraising the individual studies they determined “This review demonstrates a lack of high-quality evidence on the effects of aBME [antenatal breastmilk expression] on maternal and newborn outcomes.” (1.)
The individual studies address a wide variety of outcomes that can come from expressing milk during pregnancy. What I am most intrigued to learn about is:
How does aBME impact maternal confidence?
How does aBME impact infant health/gestational age?
There were some studies that addressed these questions. One specifically was a randomized control trial that looked at NICU rates of infants whose mothers expressed prenatally starting at 36 weeks vs the control group. (7.) I dive into this in the “I’m still curious about” section of this post. Another couple of studies looked at the maternal experience of expressing milk during pregnancy, with various answers: it appears that some mothers found it confidence boosting while others were frustrated or worried about the volume of colostrum they were able to produce while pregnant. (5, 6).
The authors of our scoping review noticed that many of the studies were conducted in the past few years, indicating increased interest in the subject. They also had some insightful recommendations for future research. It appears to me, that while there are a couple good, quality studies regarding this topic, we are still in need of future research to verify the safety and efficacy of prenatal breastmilk expression for the practice to be considered ‘evidence-based’. Go look at this scoping review for yourself, and share your thoughts in the comments.
Interesting after thought: some of the studies that were performed started pregnant women expressing as early as 20 weeks gestation. Now, the studies that started women expressing this early were from the 1940’s and 1950’s. The authors of the scoping review do caution readers regarding the interpretation of the results from these studies, as they all ranked very low in their critical appraisal.

So, after reading this and learning what a scoping review is (see “What I Learned” section”), my question is: Does the one randomized control trial regarding antenatal breastmilk expression give us enough data to guide clinical practice?
I don’t know.
Here’s what I do know:
The study recruited 635 women with pre-existing or gestational diabetes from six different hospitals in Australia. The intervention group was assigned to express breastmilk twice a day starting at 36 weeks gestation, while the control group received ‘standard care’. There was no difference between these two groups when assessing the proportion of infant admission to the NICU. The authors reported “There is no harm in advising women with diabetes in pregnancy at low risk of complications to express breastmilk from 36 weeks’ gestation.” (7.)
The authors of the scoping review did a critical appraisal of the systematic review and found …
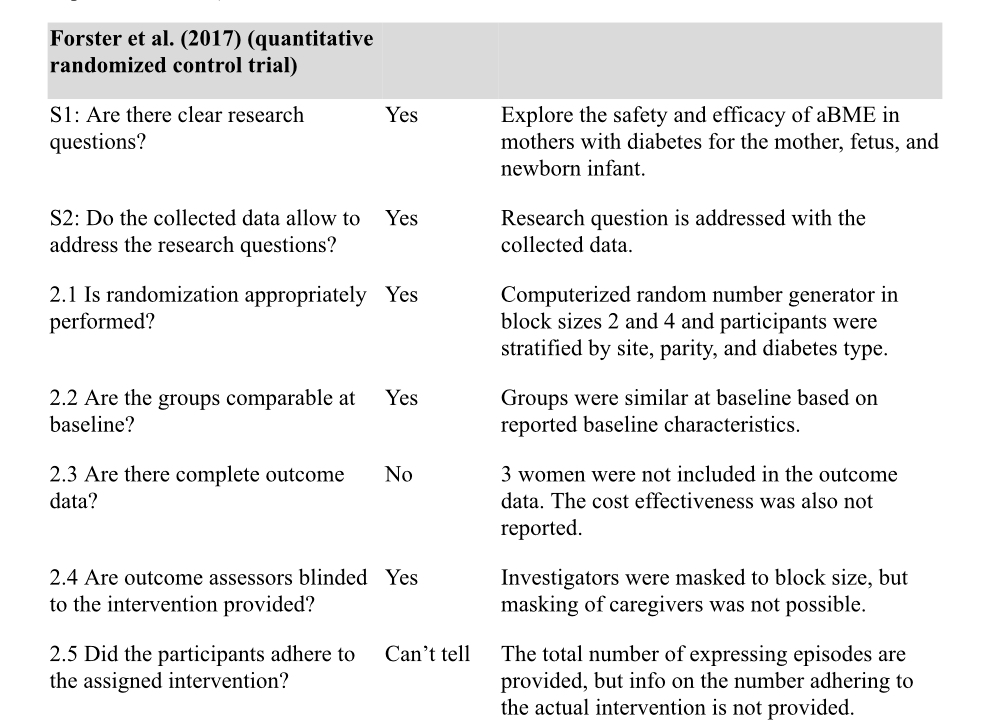
So, from this randomized control trial they determined that expressing breastmilk during pregnancy, starting at 36 weeks, did not negatively impact the infants. My question: Is this a strong enough study to recommend antenatal breastmilk expression starting at 36 weeks for low risk pregnancies?
Fun fact: there is a second RCT in progress, but I could not find published results, yet. (4.) (8.)
Readers, commenters -what do you think? I’d love to learn more from you, please leave your credentials in the comments as this will help me and our fellow readers learn. I want to hear from you whether you’ve been in the research field for 30 years or if this is your first time reading a research paper.

What I learned about after reading this scoping review:
Scoping review vs systematic review vs meta analysis
Scoping reviews (also called scoping exercises or scoping studies) are a way to synthesize evidence, and are typically used to provide an overview or map of the evidence.
Scoping reviews have many ways in which they are useful. However, they do not ask a specific question and they do not adjust for bias; therefore, scoping reviews are typically not used to inform clinical practice. Scoping reviews can be helpful in the following ways: to identify knowledge gaps, scope a body of literature, clarify concepts or to investigate research conduct. (2.)
Both systematic reviews and meta analysis can be used to inform and guide clinical practice. They “generally provide the highest level of evidence in evidence-based medicine (EBM), supporting the development and revision of clinical practice guidelines, which are recommendations for clinicians when caring for patients with specific diseases and conditions.” (3.)
A systematic review is a summary of existing published studies on a specific topic and it addresses a clearly defined question. A systematic review may, or may not, include a meta analysis. (3.)
“Systematic reviews follow a structured and pre-defined process that requires rigorous methods to ensure that the results are both reliable and meaningful to end users. These [systematic] reviews may be considered the pillar of evidence-based healthcare [15] and are widely used to inform the development of trustworthy clinical guidelines [11, 16, 17].” (2.)
A meta analysis is a mathematically driven way to combine the results from various studies. It is “a quantitative statistical analysis combining individual results to estimate the common or mean effect.” (3.)
Resources
2. Munn, Z., Peters, M.D.J., Stern, C. et al.Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 18, 143 (2018). https://doi.org/10.1186/s12874-018-0611-x
4. Demirci J. Prenatal video-based education and postpartum effects. n.d. https://clinicaltrials.gov/ct2/show/NCT04258709?cond=antenatal+breast+milk+expression&draw=2&rank=1. Accessed 11 Apr 2020.
5. Casey JRR, Mogg EL, Banks J, Braniff K, Heal C. Perspectives and experiences of collecting antenatal colostrum in women who have had diabetes during pregnancy: a North Queensland semistructured interview study. BMJ Open. 2019;9:e021513 https://doi.org/10.1136/bmjopen-2018-021513https://clinicaltrials.gov/ct2/show/NCT04258709?cond=antenatal+breast+milk+expression&draw=2&rank=1. .
7. Forster DA, Moorhead AM, Jacobs SE, Davis PG, Walker SP, McEgan KM, et al. Advising women with diabetes in pregnancy to express breastmilk in late pregnancy (Diabetes and Antenatal Milk Expressing [DAME]): a multicentre, unblinded, randomised controlled trial. Lancet. 2017;389:2204– 13 https://doi.org/10.1016/S0140-6736(17)31373-9.